Is Bcl3 Polar Or Nonpolar
The three chloride atoms have a negative charge and the one boron in the center has an equal but positive charge. Learn to determine if PF5 is polar or nonpolar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis Structure and look and.
Is SbF 3 polar or nonpolar.

Is bcl3 polar or nonpolar. That eliminates any dipole-dipole attraction called Keesom forces. Expert Answer 100 3 ratings Previous question Next question. You can use arrowsdrawingssymbols to help support your answer.
In actual fact because the molecule is symmetrical all of the dipole moments will cancel one another out. The molecular geometry of BCl3 is trigonal planar with symmetric charge distribution around the central atom. BCl3 B C l 3 has trigonal planar geometry.
Boron trichloride BCl3 is trigonal planar and has no net dipole moment it is nonpolar. Between each of the boron-chlorine pairs as caused by the large electronegativity difference between boron 204 and chlorine 316. Based on the Lewis construction CCl4 is a tetrahedral molecule.
Is CCl4 Polar Or Nonpolar. Is SbF3 Polar Or Nonpolar. Is BCl3 polar or non-polar circle your answer then using the space provided give a written explanationjustification for your choice why did you say it was non-polar or polar.
Boron trichloride or BCl3 is nonpolar. Boron sits in the center of the molecule and has three valence electrons so it balances out the three chlorides. Boron trichloride or BCl3 is nonpolar.
This is why BCl3 is considered a nonpolar molecule. Therefore this molecule is nonpolar. BCl3 is a nonpolar molecule yes and the B-Cl bonds are polar due to the electronegativity difference between the elements.
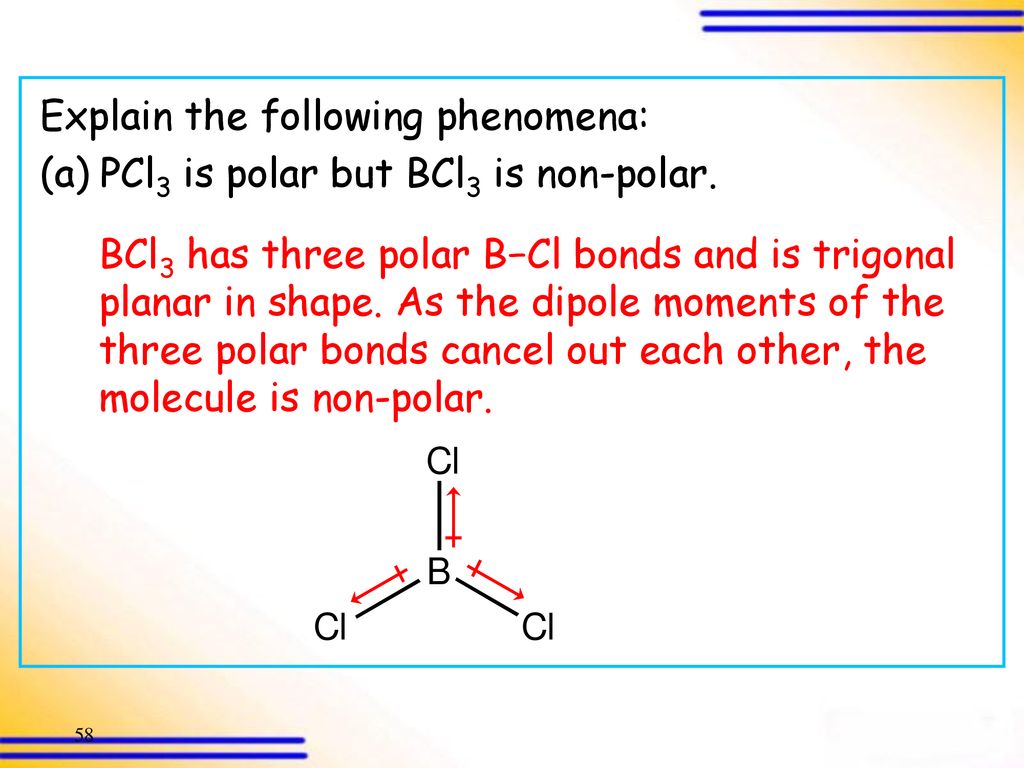
Is BCl3 Polar or Nonpolar. There are three polar B-Cl bonds in this compound but due to the symmetry in its structure all the bond dipole of polar bonds cancel each other resulting. The dipole moment of a polar molecule is always equaled to non zero and nonpolar molecules always have zero dipole moment.
Is BCl 3 polar or nonpolar. This problem has been solved. Then is CCl4 a polar covalent bond.
The electronegativity of boron is 204 and chlorine is 316. All of these bonds are polar C-H only very slightly so. The three chloride atoms have a negative charge and the one boron in the center has an equal but positive charge.
Is CCl 4 polar or nonpolar. Similar Asks Is Black Velvet good whiskey. The geometrical shape of the molecule is an important and physical parameter that helps to determine the polarity of a molecule.
BCl3 BORON TRICHLORIDE is Nonpolar. The electronegativity for C is 25 and Cl is 30 leading to a polar covalent bond. BCl3 is nonpolar due to its symmetrical structure and due to the difference of electronegativity the B-Cl bond is polar and lies at 120 degrees to each other.
The three chloride atoms have a negative charge and the one boron in the center has an equal but positive charge. Is bcl3 polar or nonpolar. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search.
In CHCl3 the molecular shape is tetrahedral meaning that the H and the three Cl atoms will occupy the vertices of a triangular based pyramid around the central C atom. This cancels out all of the different polar covalent bond pulls ie. CCl4 is an instance of a nonpolar molecule.
As a result each B-Cl bonds dipole moments cancel each other out. List molecules polar and non polar. The three chloride atoms have a negative charge and the one boron in the center has an equal but positive charge.
Boron trichloride or BCl3 is nonpolar. The bonds are arranged symmetrically around the central C atom and because the bond dipoles cancel the molecule is non-polar. Ill tell you the polar or nonpolar list below.
BCl3 is a nonpolar molecule because the chlorine halides are spaced symmetrically around the central boron atom. Is BCl3 Polar Or Nonpolar. The three bond-dipoles cancel out that is the vector sum of the individual bond dipole moments adds up to zero.
Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar. PCl3 is a polar molecule therefore its dipole moment is 097 D. This causes the optimal configuration for the chloride atoms to be a perfect triangle.
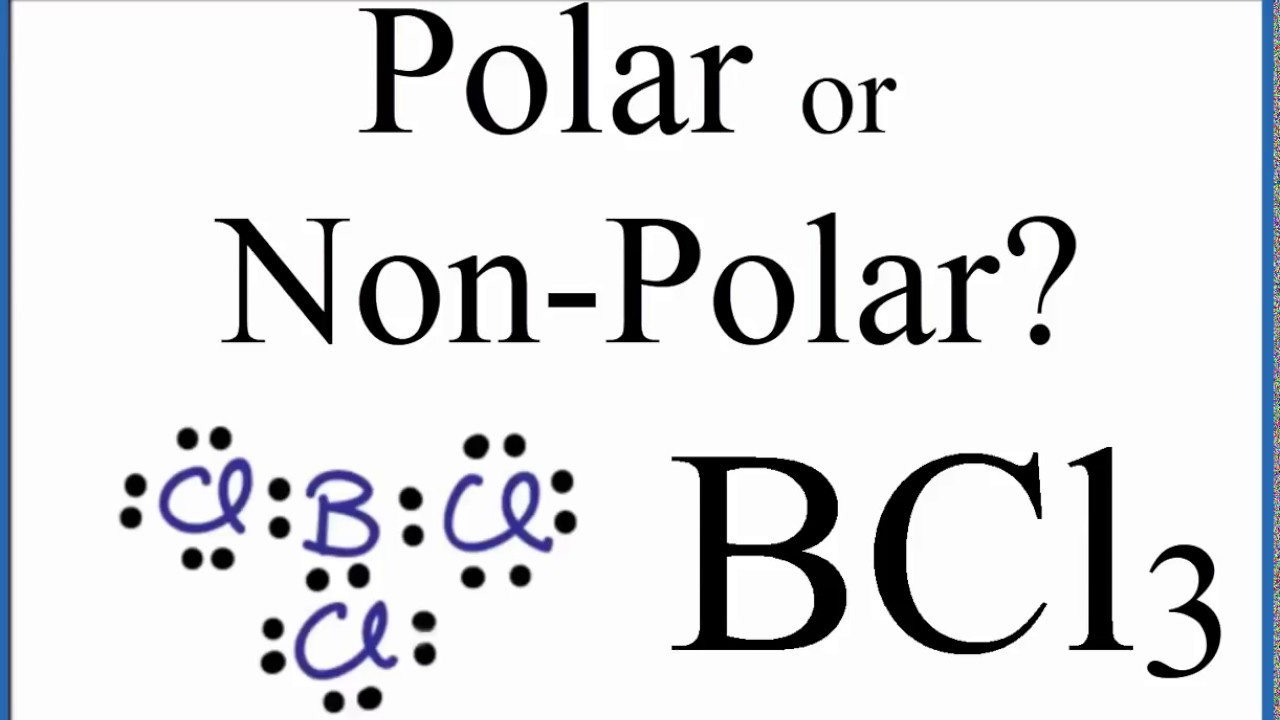
Is Bcl3 Polar Or Non Polar Boron Trichloride Youtube

Is Bcl3 Polar Or Non Polar Boron Trichloride Youtube
Is Bcl3 A Non Polar Molecule Quora

Bcl3 Is A Non Polar Molecule But Why Does It Form A Polar Bonds Quora

Is Bcl3 Polar Or Nonpolar Techiescientist

Is Bcl3 Polar Or Nonpolar All About Bcl3 Polarity Shape
Polarization Of An Ionic Bond Means The Distortion Of The Electron Cloud Of An Anion Towards A Cation Polarization Of An Ionic Bond Results In An Ionic Ppt Download


0 Response to "Is Bcl3 Polar Or Nonpolar"
Post a Comment